The following orbital diagrams show the n = 2 electrons in row 2 atoms.Which one corresponds to an excited-state atom?
A) 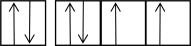
B) 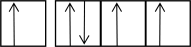
C) 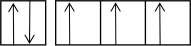
D) 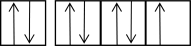
E) 
Correct Answer:
Verified
Q101: Which of the following is the ground-state
Q102: The atomic radius of germanium (Z =
Q106: What is the ground-state electron configuration of
Q111: How many unpaired electrons does the nitride
Q126: Which of the following species is NOT
Q128: Which of the following statements regarding the
Q130: Transition metal ions typically lose their outermost
Q132: How many unpaired electrons does chromium have?
A)0
B)2
C)3
D)5
E)6
Q133: Which of the following atoms or ions
Q134: Which of the following atoms has no
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents