What is the nuclide symbol for the ion that has a charge of 2+,50 neutrons more than its number of protons,and an atomic number equal to the number of electrons in a zirconium atom that has lost 2 electrons?
A) 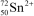
B) 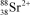
C) 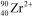
D) 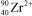
E) 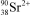
Correct Answer:
Verified
Q10: Protons and neutrons are examples of _
A)nuclei.
B)nuclides.
C)nucleons.
D)isotopes.
E)charged
Q21: Zinc oxide, a combination of zinc and
Q34: A Q36: What is the nuclide symbol for the Q37: Identify the atom or ion: i)_; ii)_; Q37: A phosphorus-31 atom has _ protons, _ Q39: What is the symbol for magnesium? Q39: Which element forms ionic compounds with the Q41: What is the charge on the manganese Q72: What is the correct formula for the![]()
A)M
B)Mg
C)Mn
D)Mo
E)Md
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents