Suppose the reaction 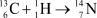 produces 1.21 10-12 J of energy (1.21 10-12 kg .m2/s2).Calculate the change in mass that occurs during the reaction in amu.E = mc2,where c = 2.998 108 m/s;
produces 1.21 10-12 J of energy (1.21 10-12 kg .m2/s2).Calculate the change in mass that occurs during the reaction in amu.E = mc2,where c = 2.998 108 m/s;
1 kg = 6.0221415 1026 amu.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q73: What is the mass in amu of
Q123: Give an example of a nonmetal.
Q124: Give an example of a metalloid also
Q137: Boron, which has an average atomic mass
Q147: How many atoms are there in 2.5
Q148: What is the average atomic mass of
Q153: Write nuclear reaction equations to show how
Q154: Hydrogen sulfide (H2S)is a highly toxic
Q155: Iron-56 has one of the highest
Q157: The carbon-nitrogen-oxygen cycle in stars is one
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents