Tetracyanoethylene has the skeleton shown below: 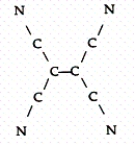 From its Lewis structure determine the following:
From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
A) 2
B) 4
C) 6
D) 8
E) 10
Correct Answer:
Verified
Q25: Tetracyanoethylene has the skeleton shown below:
Q26: Consider the skeletal structure shown below:
N-C-C-N
Draw the
Q27: Tetracyanoethylene has the skeleton shown below:
Q28: The hybridization of Br in BrF3 is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
Q29: Complete the Lewis structure for the following
Q31: The hybridization of the central atom in
Q32: Use the molecules below to answer
Q33: Whenever a set of equivalent tetrahedral atomic
Q34: Use the molecules below to answer the
Q35: When a carbon atom has sp3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents