When molten sulfur reacts with chlorine gas,a vile-smelling orange liquid forms that is found to have the empirical formula SCl.Which of the following could be the correct Lewis structure for this compound?
A) 
B) 
C) 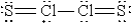
D) 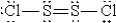
E) 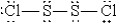 :
:
Correct Answer:
Verified
Q40: Which of the following ionic compounds has
Q41: Choose the molecule with the strongest bond.
A)F2
B)Cl2
C)Br2
D)I2
E)All
Q42: Consider the following reaction: A2 +
Q43: Which carbon in this molecule has tetrahedral
Q44: Consider the compound crotonaldehyde,whose skeleton is:
Q46: In the Lewis structure for elemental nitrogen
Q47: As the number of bonds between two
Q48: Draw the Lewis structures of the molecules
Q49: Choose the molecule with the strongest bond.
A)CH4
B)H2O
C)NH3
D)HF
E)All
Q50: Consider the compound crotonaldehyde,whose skeleton is:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents