Which of the following statements is false?
A) An orbital can accommodate at most two electrons.
B) The electron density at a point is proportional to 2 at that point.
C) The spin quantum number of an electron must be either + 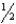 or -
or - 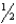 .
.
D) A 2p orbital is more penetrating than a 2s;i.e. ,it has a higher electron density near the nucleus and inside the charge cloud of a 1s orbital.
E) In the usual order of filling,the 6s orbital is filled before the 4f orbital.
Correct Answer:
Verified
Q62: The complete electron configuration of tin is
A)1s22s22p63s23p64s23d104p65s24d105d105p2
B)1s22s22p63s23p64s23d104d104p2
C)1s22s22p63s23p64s24p65s24d105d105p2
D)1s22s22p63s23p64s23d104p65s24d105p2
E)none
Q63: An atom of fluorine contains nine electrons.How
Q64: The electron configuration for the carbon atom
Q65: Of the following elements,which needs three electrons
Q66: Which of the following atoms has three
Q68: Fe has _ that is (are)unpaired in
Q69: Which of the following statements is true?
A)The
Q70: All alkaline earths have the following number
Q71: Of the following elements,which has occupied d
Q72: Which of the following statements about quantum
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents