Consider the reaction H2(g) + 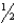 O2(g) H2O(l) H° = -286 kJ
O2(g) H2O(l) H° = -286 kJ
Which of the following is true?
A) The reaction is exothermic.
B) The reaction is endothermic.
C) The enthalpy of the products is less than that of the reactants.
D) Heat is absorbed by the system.
E) Both A and C are true.
Correct Answer:
Verified
Q19: Calculate the work for the expansion of
Q20: Which statement is true of a process
Q21: Exactly 123.7 J will raise the temperature
Q22: The enthalpy of fusion of ice is
Q23: Which one of the following statements
Q25: Consider the reaction: C2H5OH(l)+ 3O2(g)
Q26: A 32.5 g piece of aluminum (which
Q27: What is the specific heat capacity of
Q28: C2H5OH(l)+ 3O2(g)
Q29: Which of the following properties is (are)intensive
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents