Multiple Choice
The H value for the reaction 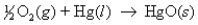 is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
A) 0.333 kJ
B) 6.07 103 kJ
C) 30.3 kJ
D) 90.8 kJ
E) none of these
Correct Answer:
Verified
Related Questions
Q54: The total volume of hydrogen gas
Q55: At 25°C,the following heats of reaction are
Q56: When 0.236 mol of a weak
Q57: The heat of combustion of benzene,C6H6,is -41.74
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents