Given: Cu2O(s) + 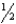 O2(g) 2CuO(s) H° = -144 kJ
O2(g) 2CuO(s) H° = -144 kJ
Cu2O(s) Cu(s) + CuO(s) H° = +11 kJ
Calculate the standard enthalpy of formation of CuO(s) .
A) -166 kJ
B) -299 kJ
C) +299 kJ
D) +155 kJ
E) -155 kJ
Correct Answer:
Verified
Q60: A bomb calorimeter has a heat
Q61: _ involves the transfer of energy between
Q62: Choose the correct equation for the
Q63: Which of the following is both a
Q64: Consider the reaction:
N2(g)+ 3H2(g)
Q66: This fossil fuel was formed from the
Q67: Consider the reaction: 2ClF3(g)+ 2NH3(g)
Q68: The _ of a system is the
Q69: The heat combustion of acetylene,C2H2(g),at 25°C
Q70: The coal with the highest energy available
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents