Consider the Reaction:
N2(g)+ 3H2(g) 2NH3(g)
Assuming This Reaction Takes Place in an Elastic Balloon
Consider the reaction:
N2(g)+ 3H2(g) 2NH3(g)
Assuming this reaction takes place in an elastic balloon with an atmospheric pressure of 1.0 atm,and that you have a stoichiometric mixture of nitrogen and hydrogen,draw a microscopic diagram before and after the reaction occurs.See the example below to assist you.
ABC2(g) AB(g)+ C2(g)(could be drawn as) 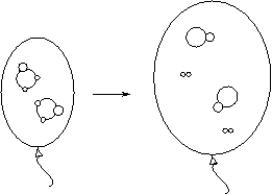
In addition,explain whether w (the work done)is positive,negative,or zero.
Correct Answer:
Verified
Work (w)will be positive since the sur...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q59: The
Q60: A bomb calorimeter has a heat
Q61: _ involves the transfer of energy between
Q62: Choose the correct equation for the
Q63: Which of the following is both a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents