Consider the following data: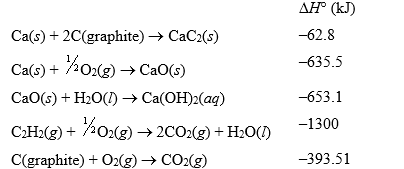
Use Hess' law to find the change in enthalpy at 25°C for the following equation:
CaC2(s)+ 2H2O(l) Ca(OH)2(aq)+ C2H2(g)
Correct Answer:
Verified
Q76: One of the main advantages of hydrogen
Q77: Using the information below,calculate
Q78: For the reaction: AgI(s)+ 
Q79: Which of the following is not being
Q80: The combustion of hydrogen gas releases 286
Q82: Consider the following reaction:
2Al(s)+ 3Cl2(g)
Q83: Acetylene (C2H2)and butane (C4H10)are gaseous fuels.Determine the
Q84: To carry out the reaction N2
Q85: Consider the following standard heats of
Q86: To carry out the reaction N2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents