Consider a sample of helium gas in a container fitted with a piston,as pictured below.The piston is frictionless,but has a mass of 10.0 kg.How many of the following processes will cause the piston to move away from the base and decrease the pressure of the gas? Assume ideal behavior.
10.0 kg
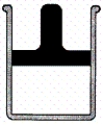
base
I.heating the helium
II.removing some of the helium from the container
III.turning the container on its side
IV.decreasing the pressure outside the container
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:
Verified
Q1: You have 41.6 g of O2 gas
Q2: A glass column is filled with mercury
Q3: You have a certain mass of helium
Q4: Gases generally have
A)low density
B)high density
C)closely packed particles
D)no
Q6: Charles's law states that:
A)Equal amounts of gases
Q7: You are holding two balloons,an orange balloon
Q9: A gas sample is held at constant
Q10: Boyle's law states that:
A)Equal amounts of gases
Q11: Consider a sample of gas in a
Q17: The local weather forecaster reports that the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents