A 3.54 gram sample of a certain diatomic gas occupies a volume of 3.30-L at 1.00 atm and a temperature of 45°C.Identify this gas.
A) 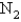
B) 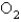
C) 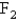
D) 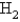
E) 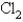
Correct Answer:
Verified
Q55: The bag is emptied and refilled,successively,with gases
Q56: Four identical 1.0-L flasks contain the gases
Q57: Four identical 1.0-L flasks contain the gases
Q58: Given reaction N2 + 3H2
Q59: A plastic bag is weighed and then
Q61: At 1000°C and 10.torr,the density of a
Q62: An excess of sodium hydroxide is treated
Q63: Air has an average molar mass of
Q64: The purity of a sample containing zinc
Q65: A 1.00-g sample of a gaseous compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents