The purity of a sample containing zinc and weighing 0.312 g is determined by measuring the amount of hydrogen formed when the sample reacts with an excess of hydrochloric acid.The determination shows the sample to be 84.0% zinc.What amount of hydrogen (measured at STP) was obtained?
A) 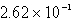 L
L
B) 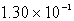 g
g
C) 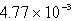 mole
mole
D) 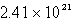 molecules
molecules
E) 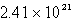 atoms
atoms
Correct Answer:
Verified
Q42: Into a 2.22-liter container at 25°C are
Q59: A plastic bag is weighed and then
Q60: A 3.54 gram sample of a certain
Q61: At 1000°C and 10.torr,the density of a
Q62: An excess of sodium hydroxide is treated
Q63: Air has an average molar mass of
Q65: A 1.00-g sample of a gaseous compound
Q67: Standard pressure for gases is
A)0 atm
B)1 atm
C)100
Q68: One way to isolate metals from their
Q69: A 3.31-g sample of lead nitrate,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents