The valve between the 2.00-L bulb,in which the gas pressure is 1.80 atm,and the 3.00-L bulb,in which the gas pressure is 3.00 atm,is opened.What is the final pressure in the two bulbs,the temperature remaining constant? 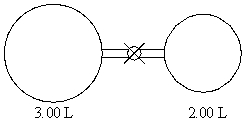
A) 0.720 atm
B) 2.28 atm
C) 2.52 atm
D) 1.80 atm
E) 2.40 atm
Correct Answer:
Verified
Q69: A vessel with a volume of 26.9
Q94: A balloon contains an anesthetic mixture of
Q95: Zinc metal is added to hydrochloric acid
Q96: Calculate the root mean square velocity for
Q97: A 142-mL sample of gas is collected
Q98: In the kinetic molecular theory we assume
Q101: A sample of N2 gas is contaminated
Q102: Which of the following statements is true
Q103: All the following are postulates of the
Q104: Complete the following: Because real gas particles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents