The rate of effusion of an unknown gas was measured and found to be 11.9 mL/min.Under identical conditions,the rate of effusion of pure oxygen (O2) gas is 14.0 mL/min.Based on this information,the identity of the unknown gas could be:
A) 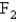
B) 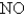
C) 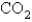
D) 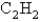
E) none of these
Correct Answer:
Verified
Q101: A sample of N2 gas is contaminated
Q102: Which of the following statements is true
Q103: All the following are postulates of the
Q104: Complete the following: Because real gas particles
Q105: Which of the following would have a
Q107: According to the postulates of the kinetic
Q108: Which of the following is true about
Q109: Graham's law states that:
A)Equal amounts of gases
Q110: Order the following in increasing rate of
Q111: Which of the following is included as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents