Combustion of coal releases sulfur dioxide into the atmosphere.The following process converts this gas into sulfuric acid,a component of acid rain. 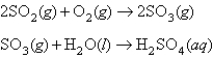 If each tonne of coal produces
If each tonne of coal produces 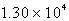 L of sulfur dioxide (measured at STP) ,what mass of sulfuric acid can result from combustion of each tonne of coal? (1 tonne = 1000 kg)
L of sulfur dioxide (measured at STP) ,what mass of sulfuric acid can result from combustion of each tonne of coal? (1 tonne = 1000 kg)
A) 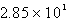 kg H2SO4
kg H2SO4
B) 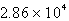 kg H2SO4
kg H2SO4
C) 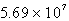 kg H2SO4
kg H2SO4
D) 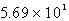 kg H2SO4
kg H2SO4
E) 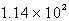 kg H2SO4
kg H2SO4
Correct Answer:
Verified
Q77: Real gases are those that
A) only behave
Q116: Which of the following properties of a
Q117: The diffusion of a gas is faster
Q118: Consider the following containers,one with helium at
Q119: Calculate the ratio of the effusion rates
Q120: At the same temperature,lighter molecules have a
Q122: The pressure a gas would exert under
Q124: What is the name for the lowest
Q125: Toy balloons are filled with hydrogen gas,at
Q126: Which of the following statements is least
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents