With what volume of 5.00 M HF will 4.72 g of calcium hydroxide react completely,according to the following reaction? 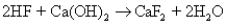
A) 12.7 mL
B) 127 mL
C) 637 mL
D) 25.5 mL
E) 39.2 mL
Correct Answer:
Verified
Q64: When solutions of carbonic acid and aluminum
Q65: When solutions of carbonic acid and potassium
Q66: A 0.307-g sample of an unknown triprotic
Q67: Sulfamic acid,HSO3NH2 (molar mass = 97.1 g/mol),is
Q68: When solutions of carbonic acid and copper(II)hydroxide
Q70: You have 88.6 mL of a 2.50
Q71: What mass of NaOH is required to
Q72: A 3.00-g sample of an alloy (containing
Q73: You have 75.0 mL of a 2.50
Q74: You have 75.0 mL of a 2.50
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents