The major industrial source of hydrogen gas is the reaction of methane and water at high temperatures (800 - 1000°C) and high pressures (10 - 15 atm) with nickel as a catalyst. CH4(g) + H2O(g) 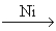 CO2(g) + 3H2(g)
CO2(g) + 3H2(g)
If 171.5 g of CH4 and 171.5 g of H2O are reacted at 945°C and 11.0 atm,how much hydrogen should be available for industrial use?
A) 201 L
B) 291 L
C) 259 L
D) 28.8 L
E) 86.5 L
Correct Answer:
Verified
Q35: Which group contains the most active metals?
A)Group
Q36: The strongest reducing agent in the alkali
Q37: The compound SiO2 does not exist as
Q38: Both CO2 and SiO2 appear to have
Q39: The elements in this group are termed
Q41: Choose the solid that has the smallest
Q42: Arrange the following Group 2A elements (in
Q43: The ion that aluminum is most likely
Q44: Which of the following ions interferes with
Q45: The element with the lowest melting point
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents