The half-life of 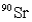 is 28.1 years.How long will it take a 10.0-g sample of
is 28.1 years.How long will it take a 10.0-g sample of 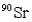 to decompose to 0.23 g?
to decompose to 0.23 g?
A) 102 years
B) 306 years
C) 153 years
D) 229 years
E) none of these
Correct Answer:
Verified
Q23: Consider a certain type of nucleus
Q24: The number of a certain radioactive nuclide
Q25: The number of half-lives needed for a
Q26: The Cs-131 nuclide has a half-life of
Q27: The half-life of a sample has been
Q29: The rate constant for the beta
Q30: The rate constant for the decay
Q31: Which types of processes are likely
Q32: The half-life of Q33: A radioactive sample has an initial![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents