Calculate E in kilojoules per mole for the following reaction: 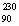 Th
Th 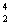 He +
He + 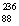 Ra Atomic masses: 230Th = 230.0332,4He = 4.00260,226Ra = 226.02544.
Ra Atomic masses: 230Th = 230.0332,4He = 4.00260,226Ra = 226.02544.
A) -4.6 108 kJ/mol
B) -2.4 106 kJ/mol
C) 0
D) +2.4 106 kJ/mol
E) +4.6 108 kJ/mol
Correct Answer:
Verified
Q44: One of the hopes for solving
Q45: Identify the missing particle in the
Q46: Consider the following process: 
Q47: An archaeological sample contains 0.739 g
Q48: If a tree dies and the trunk
Q50: It is desired to determine the
Q51: Radioactive tracers are useful in studying
Q52: The I-131 nuclide has a half-life of
Q53: A sample of wood from an Egyptian
Q54: The half-life for electron capture for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents