Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) : 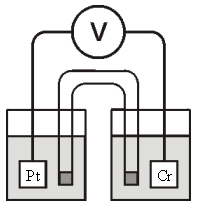 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
The standard reduction potentials are as follows:
Cr3+(aq) + 3e- Cr(s) =-0.73 V
Br2(aq) + 2e- 2Br-(aq) = +1.09 V
Which of the following statements about this cell is false?
A) This is a galvanic cell.
B) Electrons flow from the Pt electrode to the Cr electrode.
C) Reduction occurs at the Pt electrode.
D) The cell is not at standard conditions.
E) To complete the circuit,cations migrate into the left half-cell and anions migrate into the right half-cell from the salt bridge.
Correct Answer:
Verified
Q26: Which of the following reactions is
Q27: Which of the following is the
Q28: What is the oxidation state of Cr
Q29: Consider a galvanic cell based in
Q30: Which of the following is true for
Q32: Which statement is always true of the
Q33: Consider the galvanic cell shown below
Q34: Which of the following is the
Q35: Refer to the galvanic cell below
Q36: In which direction do electrons flow in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents