Choose the correct statement given the following information: 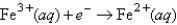
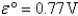
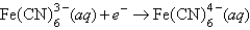
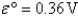
A) Fe2+(aq) is more likely to be oxidized than Fe2+ complexed to CN-.
B) Fe3+(aq) is more likely to be reduced than Fe3+ complexed to CN-.
C) Both A and B are true.
D) Complexation of Fe ions with CN- has no effect on their tendencies to become oxidized or reduced.
E) None of these is true.
Correct Answer:
Verified
Q42: In the balanced cell reaction,what is the
Q43: The following question refers to the
Q44: Consider the following electrode potentials: Mg2+
Q45: You wish to plate out zinc metal
Q46: Of Sn2+,Ag+,and/or Zn2+,which could be reduced by
Q48: Which of the following would be the
Q49: What is the cell reaction for the
Q50: How many electrons are transferred in the
Q51: What is the cell potential at 25°C
Q52: What is the balanced chemical equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents