Determine the standard potential, ,of a cell that employs the reaction: Fe + Cu2+ Cu + Fe2+.
Reaction
(volts) 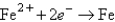 -0.441
-0.441 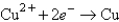 +0.340
+0.340
A) -0.101
B) -0.781
C) 0.101
D) 0.781
E) -0.202
Correct Answer:
Verified
Q36: In which direction do electrons flow in
Q37: Which metal,Al or Ni could reduce
Q38: You are told that metal X is
Q39: Refer to the galvanic cell below
Q40: Which of the following species cannot function
Q42: In the balanced cell reaction,what is the
Q43: The following question refers to the
Q44: Consider the following electrode potentials: Mg2+
Q45: You wish to plate out zinc metal
Q46: Of Sn2+,Ag+,and/or Zn2+,which could be reduced by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents