The reduction potentials for Au3+ and Ni2+ are as follows: Au3+ + 3e- Au, = +1.50 V
Ni2+ + 2e- Ni, = -0.229 V
Calculate G° (at 25°C) for the reaction:
2Au3+ + 2Ni 3Ni2+ + 2Au
A) 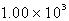 kJ
kJ
B) 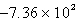 kJ
kJ
C) 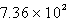 kJ
kJ
D) 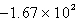 kJ
kJ
E) 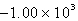 kJ
kJ
Correct Answer:
Verified
Q63: A fuel cell designed to react
Q64: Consider the following reduction potentials: Cu2+
Q65: Which of the following statements is
Q66: Determine
Q67: Which of the following cell reactions
Q69: For a certain reaction,
Q70: What is
Q71: A common car battery consists of
Q72: Under standard conditions,which of the following operations
Q73: A galvanic cell consists of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents