A common car battery consists of six identical cells,each of which carries out the reaction: Pb + PbO2 + 2HSO4- + 2H+ 2PbSO4 + 2H2O
The value of 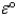 for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.
for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.
A) -196.7 kJ
B) -98.37 kJ
C) -393.5 kJ
D) -786.9 kJ
E) -590.2 kJ
Correct Answer:
Verified
Q66: Determine
Q67: Which of the following cell reactions
Q68: The reduction potentials for Au3+ and
Q69: For a certain reaction,
Q70: What is
Q72: Under standard conditions,which of the following operations
Q73: A galvanic cell consists of
Q74: Tables of standard reduction potentials are
Q75: For a reaction in a voltaic
Q76: Consider an electrochemical cell with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents