The following question refers to the following system: 3Ag(s) + NO3-(aq) + 4H+(aq) 3Ag+(aq) + NO(g) + 2H2O(l)
Anode reaction:
Ag Ag+(aq) + 1e-
° = -0.7990 V
Cathode reaction:
NO3-(aq) + 4H+(aq) + 3e- NO(g) + 2H2O(l)
° = 0.9636 V
Determine the equilibrium constant at 25°C.
A) 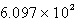
B) 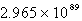
C) 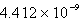
D) 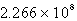
E) 3.117
Correct Answer:
Verified
Q56: Copper will spontaneously reduce which of the
Q57: The galvanic cell described by Zn(s)|
Q58: A cell is set up with
Q59: The following has a potential of
Q60: Which of the following cell diagrams
Q62: For a particular reaction in a
Q63: A fuel cell designed to react
Q64: Consider the following reduction potentials: Cu2+
Q65: Which of the following statements is
Q66: Determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents