The reduction potentials for Ni2+ and Sn2+ are as follows: Ni2+ + 2e- Ni, ° = -0.231 V
Sn2+ + 2e- Sn, ° = -0.140 V
Calculate the equilibrium constant at 25 °C for the reaction:
Sn2+ + Ni  Sn + Ni2+
Sn + Ni2+
A) 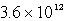
B) 35
C) 5.9
D) 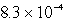
E) 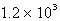
Correct Answer:
Verified
Q86: If a reducing agent M reacts
Q87: Calculate the solubility product of silver
Q88: The equilibrium constant at 25°C for the
Q89: Which of the following statements is
Q90: You make a cell with an aluminum
Q92: Consider the galvanic cell shown below
Q93: An excess of finely divided iron is
Q94: A concentration cell is constructed using
Q95: Consider an electrochemical cell with a
Q96: Using the following data to calculate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents