What quantity of charge is required to reduce 27.8 g of CrCl3 to chromium metal? (1 faraday = 96,485 coulombs)
A) 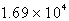 C
C
B) 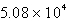 C
C
C) 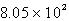 C
C
D) 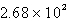 C
C
E) none of these
Correct Answer:
Verified
Q102: Which type of battery has been designed
Q103: An antique automobile bumper is to be
Q104: Which of the following statements is false?
A)Stainless
Q105: How many faradays are involved in conversion
Q106: Which of the following statements about corrosion
Q108: What is
Q109: How many moles of electrons are
Q110: For the cell Cu(s)| Cu2+ || Ag+
Q111: A common car battery consists of
Q112: An antique automobile bumper is to be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents