A solution of MnO42- is electrolytically reduced to Mn3+.A current of 8.07 amp is passed through the solution for 15.0 minutes.What is the number of moles of Mn3+ produced in this process? (1 faraday = 96,485 coulombs)
A) 0.0753
B) 0.226
C) 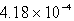
D) 0.0251
E) 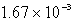
Correct Answer:
Verified
Q124: If an electrolysis plant operates its
Q125: Balance the following equation: MnO4- +
Q126: What mass of Ti(s)may be deposited
Q127: If a constant current of 5.0 amperes
Q128: An unknown metal (M)is electrolyzed.It took 74.1
Q130: Consider a galvanic cell with a
Q131: Nickel is electroplated from a NiSO4 solution.A
Q132: Gold (atomic mass = 197.0)is plated from
Q133: What mass of chromium could be deposited
Q134: What are the products of the chlor-alkali
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents