Which of the following result(s) in an increase in the entropy of the system?
I. 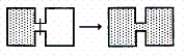
II.
Br2(g) Br2(l)
III.
NaBr(s) Na+(aq) + Br-(aq)
IV.
O2(298 K) O2(373 K)
V.
NH3(1 atm,298 K) NH3(3 atm,298 K)
A) I
B) II,V
C) I,III,IV
D) I,II,III,IV
E) I,II,III,V
Correct Answer:
Verified
Q1: Consider the following processes:
I.condensation of a
Q2: Which of the following statements is true?
A)The
Q3: Which of the following shows a decrease
Q4: Assume that the enthalpy of fusion of
Q6: The heat of vaporization for 1.0
Q7: A two-bulbed flask contains 5 particles.What is
Q8: Ten identical coins are shaken vigorously in
Q9: A change of state that occurs
Q10: A 100-mL sample of water is
Q11: If two pyramid-shaped dice (with numbers 1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents