Multiple Choice
Consider two perfectly insulated vessels.Vessel #1 initially contains an ice cube at 0°C and water at 0°C.Vessel #2 initially contains an ice cube at 0°C and a saltwater solution at 0°C.In each vessel,consider the "system" to be the ice,and the "surroundings" to be the liquid.
-Determine the sign of Ssys, Ssurr,and Suniv for the contents of Vessel #1.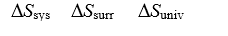
A) 0 0 0
B) + - 0
C) + + +
D) + - +
E) + 0 +
Correct Answer:
Verified
Related Questions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents