Consider the freezing of liquid water at -10°C.For this process what are the signs for H, S,and G? 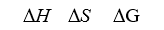
A) + - 0
B) + - -
C) - + 0
D) - + -
E) - - -
Correct Answer:
Verified
Q47: The following reaction takes place at
Q48: A mixture of hydrogen and chlorine
Q49: The following questions refer to the
Q50: For the reaction A + B
Q51: When ignited,solid ammonium dichromate decomposes in
Q53: When ignited,solid ammonium dichromate decomposes in
Q54: At constant pressure,the following reaction 2NO2(g)
Q55: The third law of thermodynamics states:
A)The entropy
Q56: For the process of a certain
Q57: In which case must a reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents