The equilibrium constant of a certain reaction was measured at various temperatures to give the plot shown below.What is S° for the reaction in J/mol . K? 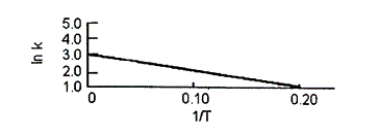
A) 0.20
B) 3.0
C) 25
D) -50.
E) -8.3 103
Correct Answer:
Verified
Q107: Consider a weak acid,HX.If a 0.10
Q108: For the reaction CO(g)+ 2H2(g)
Q109: For the reaction 2HF(g) 
Q110: For a certain process,at 300.K
Q111: Consider the following system at equilibrium
Q112: Which statement is true?
A)All real processes are
Q113: Consider the following system at equilibrium
Q115: The equilibrium constant Kp (in atm)for
Q116: Consider the following system at equilibrium
Q117: Would you predict an increase or decrease
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents