The Ksp of PbSO4 is 1.3 10-8.Calculate the solubility (in mol/L) of PbSO4 in a 0.0037 M solution of Na2SO4.
A) 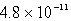 M
M
B) 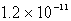 M
M
C) 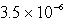 M
M
D) 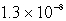 M
M
E) 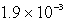 M
M
Correct Answer:
Verified
Q26: The Ksp of an unknown salt,MZ2,is
Q27: Which of the following compounds has
Q28: The solubility of an unknown salt,M2Z,in a
Q29: The solubility of silver phosphate,Ag3PO4,at 25°C
Q30: Calculate the solubility of Ca3(PO4)2 (Ksp
Q32: Which of the following compounds has
Q33: How many moles of Fe(OH)2 [Ksp
Q34: The solubility of Mg(OH)2 (Ksp =
Q35: The molar solubility of AgCl (Ksp
Q36: In a solution prepared by adding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents