Chromate ion is added to a saturated solution of Ag2CrO4 to reach 0.78 M CrO42-.Calculate the final concentration of silver ion at equilibrium (Ksp for Ag2CrO4 is 9.0 10-12) .
A) 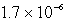 M
M
B) 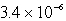 M
M
C) 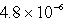 M
M
D) 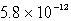 M
M
E) 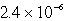 M
M
Correct Answer:
Verified
Q17: An unknown salt,M3Z,has a Ksp of
Q18: The concentration of OH- in a
Q19: Barium carbonate has a measured solubility
Q20: Find the solubility (in mol/L)of lead(II)chloride,PbCl2,at
Q21: Calculate the solubility of Ag2CrO4 (Ksp
Q23: The solubility of La(IO3)3 in a 0.42
Q24: Solubility Products (Ksp) Q25: Which of the following salts shows Q26: The Ksp of an unknown salt,MZ2,is Q27: Which of the following compounds has![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents