What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 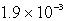 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
A) 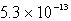 M
M
B) 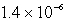 M
M
C) 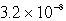 M
M
D) 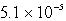 M
M
E) None of these;MgCO3 will always precipitate before BaCO3.
Correct Answer:
Verified
Q71: A 0.012-mol sample of Na2SO4 is
Q72: Consider a solution containing the following cations:
Q73: In the classic scheme for qualitative analysis,the
Q74: Sodium chloride is added slowly to
Q75: What is the maximum concentration of
Q77: If 30 mL of 5.0
Q78: A 100.-mL sample of solution contains
Q79: In the qualitative analysis scheme for metal
Q80: A solution contains 0.018 moles each of
Q81: Consider a solution made by mixing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents