What is the concentration of the Cd2+(aq) ion in a 0.030 M Cd(NO3) 2 solution that is also 1.0 M NH3? At this temperature,Kf for Cd(NH3) 42+ = 1.0 107.
A) 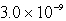 M
M
B) 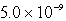 M
M
C) 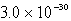 M
M
D) 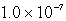 M
M
E) none of these
Correct Answer:
Verified
Q78: A 100.-mL sample of solution contains
Q79: In the qualitative analysis scheme for metal
Q80: A solution contains 0.018 moles each of
Q81: Consider a solution made by mixing
Q82: Given the following values of equilibrium
Q84: The following questions refer to the
Q85: The following questions refer to the
Q86: Consider a solution made by mixing
Q87: The following questions refer to the
Q88: Calculate the molar concentration of uncomplexed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents