Consider the Following Information About the Diprotic Acid,ascorbic Acid 10-5)
HAs- As2- + H+ pKa
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As  HAs- + H+ pKa
HAs- + H+ pKa
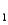 = 4.10 (Ka
= 4.10 (Ka
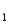 = 7.9 10-5)
= 7.9 10-5)
HAs-  As2- + H+ pKa
As2- + H+ pKa
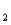 = 11.79 (Ka
= 11.79 (Ka
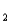 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: 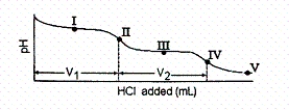
-What is the pH at point I (V1/2 HCl added) ?
A) 4.10
B) 7.95
C) 11.79
D) 12.39
E) none of these
Correct Answer:
Verified
Q63: You have 100.0 mL of 0.100
Q64: A 50.00-mL solution of 0.0350 M
Q65: A 100.0-mL sample of 0.2 M
Q66: How many of the following will raise
Q67: Consider the following information about the
Q69: Calculate the pH at the equivalence point
Q70: Consider the titration of 100.0 mL
Q71: What volume of 0.0100 M NaOH
Q72: You dissolve 1.15 grams of an unknown
Q73: Which of the following is a major
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents