A solution containing 10.mmol of 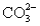 and 5.0 mmol of
and 5.0 mmol of 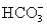 is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
A) 2.8 mL
B) 5.6 mL
C) 10.6 mL
D) 15.6 mL
E) 20.6 mL
Correct Answer:
Verified
Q73: Which of the following is a major
Q74: A 2.36-g sample of an acid,H2X,requires 45.0
Q75: A 25.00-mL sample of propanoic acid,CH3CH2COOH,of unknown
Q76: Which of the following is the
Q77: Consider the titration of 100.0 mL
Q79: A 0.210-g sample of an acid (molar
Q80: In titrating 100 mL of 0.10 M
Q81: Consider the titration of 100.0 mL of
Q82: In the titration of a weak acid
Q83: A 50.00-mL solution of 0.0304 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents