Given the following acids and Ka values: 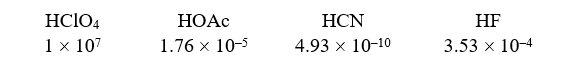
What is the order of increasing base strength?
A) CN-,F-,OAc-,ClO4-
B) CN-,OAc-,F-,ClO4-
C) CN-,ClO4-,F-,OAc-
D) ClO4-,OAc-,CN-,F-
E) ClO4-,F-,OAc-,CN-
Correct Answer:
Verified
Q9: What is the equilibrium constant for
Q10: Assuming that the value for K in
Q11: The equilibrium constant for the reaction A-
Q12: Consider the reaction HOCl + F-
Q13: The following three equations represent equilibria that
Q15: Which of the following is a conjugate
Q16: HA and HB are both weak acids
Q17: Consider the reaction HNO2(aq)+ H2O(l) 
Q18: The hydrogen sulfate or bisulfate ion
Q19: Which of the following is the equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents