If,at a given temperature,the equilibrium constant for the reaction H2(g) + Cl2(g)  2HCl(g) is Kp,then the equilibrium constant for the reaction HCl(g)
2HCl(g) is Kp,then the equilibrium constant for the reaction HCl(g) 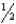 H2(g) +
H2(g) + 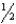 Cl2 (g) can be represented as:
Cl2 (g) can be represented as:
A) 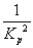
B) Kp2
C) 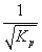
D) 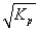
E) none of these
Correct Answer:
Verified
Q1: The value of the equilibrium constant,K,is dependent
Q2: Determine the equilibrium constant for the
Q3: Apply the law of mass action to
Q4: For the reaction N2O4(g) 
Q6: Which of the following is true about
Q7: Equilibrium is reached in chemical reactions when:
A)The
Q8: Given the equilibrium constants for the following
Q9: Consider the chemical system CO +
Q10: For the reaction H2(g)+ Cl2(g)
Q11: Consider the gaseous reaction CO(g)+ Cl2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents