When ethyl chloride,CH3CH2Cl,is dissolved in 1.0 M NaOH,it is converted into ethanol,CH3CH2OH,by the reaction: 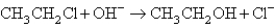 At 25°C the reaction is first order in CH3CH2Cl,and the rate constant is 3.1 10-3 s-1.If the activation parameters are A = 3.4 1014 s-1 and Ea = 100.0 kJ/mol,what will the rate constant be at 40.°C?
At 25°C the reaction is first order in CH3CH2Cl,and the rate constant is 3.1 10-3 s-1.If the activation parameters are A = 3.4 1014 s-1 and Ea = 100.0 kJ/mol,what will the rate constant be at 40.°C?
A) 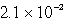 s-1
s-1
B) 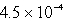 s-1
s-1
C) 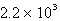 s-1
s-1
D) 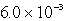 s-1
s-1
E) 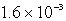 s-1
s-1
Correct Answer:
Verified
Q97: According to collision theory,the activated complex that
Q98: The reaction: 2A + B
Q99: The rate constant k is dependent on
I.the
Q100: What would happen if the kinetic energy
Q101: Which of the following statements about enzymes
Q103: Which of the following statements best describes
Q104: For the second-order reaction NO(g)+ O3(g)
Q105: The rate constant for a reaction
Q106: The reaction 2H2O2
Q107: The catalyzed pathway in a reaction mechanism
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents