Consider the representations below to answer the next three questions.
(I) 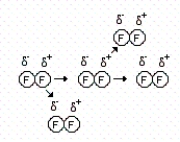 (II)
(II) 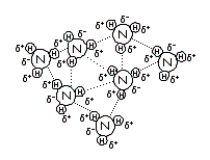 (III)
(III) 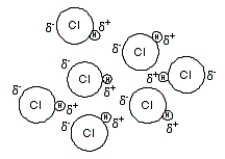
-How many of the following statements are correct concerning drawing I?
I.Each molecule induces a dipole onto the next molecule in close proximity.
II.The phenomenon shown is relatively weak and short-lived.
III.C8H18 contains this type of interaction.
IV.The forces that exist in this example are London dispersion forces.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:
Verified
Q9: The bonds between hydrogen and oxygen within
Q11: Which of the following is the correct
Q12: Hydrogen bonding is a type of London
Q13: In general,the density of a compound as
Q15: Which of the following would you expect
Q16: Order the intermolecular forces (dipole-dipole,London dispersion,ionic,and hydrogen-bonding)from
Q17: Second row hydrides generally have higher than
Q18: Hydrogen bonds account for which of the
Q19: Which substance involves no bonding forces except
Q20: When a water molecule forms a hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents