Aluminum metal crystallizes in a face-centered cubic structure.The relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E) is:
A) r = E/2
B) 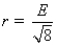
C) 
D) r = 2E
E) 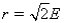
Correct Answer:
Verified
Q31: Which of the following statements is (are)false?
I.The
Q32: Methane (CH4)exhibits stronger hydrogen bond interactions than
Q33: When a nonpolar liquid displays a convex
Q34: Atomic solids generally have low melting points.
Q35: Chromium metal crystallizes as a body-centered cubic
Q37: Table salt and table sugar are both
Q38: A crystal was analyzed with x-rays
Q39: The volume of a single cell.
A)1.20
Q40: What is responsible for capillary action,a property
Q41: What is the simplest formula of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents