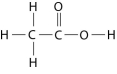
 Figure 2.10
Figure 2.10
-How many grams of the compound in Figure 2.10 would be required to make 2.5 L of a 1 M solution? (carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:
Verified
Q61: A slice of pizza has 500 kcal.
Q65: You have two beakers. One contains a
Q67: We can be sure that a mole
Q80: Which of the following statements correctly describes
Q125: A group of molecular biologists is trying
Q127: What coefficients must be placed in the
Q128: A small birthday candle is weighed. It
Q131: Carbon dioxide (CO2) is readily soluble in
Q132: Identical heat lamps are arranged to shine
Q135: The atomic number of sulfur is 16.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents