Which statement concerning the crystal field theory of octahedral transition metal complexes is not correct?
A) The crystal field splitting ( o) increases with increasing charge of the metal ion for the same set of ligands.
B) The crystal field splitting ( o) is determined solely by the charge of the transition metal complex ion.
C) The 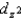 and
and 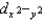 orbitals are higher in energy than the dxy, dxz, and dyz orbitals because these two point directly toward the ligands.
orbitals are higher in energy than the dxy, dxz, and dyz orbitals because these two point directly toward the ligands.
D) All ligands contribute to the strength of the crystal field.
E) The ordering of the orbitals, 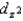 and
and 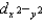 are higher in energy than the dxy, dxz, and dyz orbitals, is independent of the strength of the crystal field.
are higher in energy than the dxy, dxz, and dyz orbitals, is independent of the strength of the crystal field.
Correct Answer:
Verified
Q57: Crystal field theory describes _
A)how ligands cause
Q58: Which statement describing the splitting of metal
Q59: Which statement concerning ligands in transition metal
Q60: Complex ions with different ligands have different
Q61: Which of the following ligands will cause
Q63: Which of the following ligands will cause
Q64: How many d electrons are there in
Q65: How many unpaired spins are there in
Q66: Which d orbital(s) is/are highest in energy
Q67: Which d orbital(s) is/are lowest in energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents