Porphyrins are polydentate ligands found in nature. They support the iron centers in heme proteins, which allows for the transport of oxygen throughout the blood stream. A structure of the core of a porphyrin is shown below. Based on the structure, what type of denticity would the ligand likely possess? 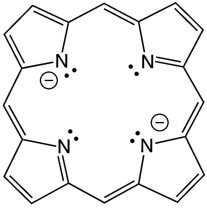
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q122: Define the term polydentate ligand, and give
Q123: What is the correct formula for hexamminecobalt(III)
Q124: What is the correct formula for pentaammineaquairon(II)
Q125: Draw and label (cis and trans) the
Q126: Why are compounds of most of the
Q128: What is the name of [Fe(NH3)4(H2O)2][NiCl4]?
Q129: What determines whether a transition metal ion
Q130: How many unpaired electrons are there in
Q131: Explain why Ni(CN)42- is diamagnetic and NiCl42-
Q132: What is the name of [Fe(NH3)5Br]2+?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents