Tooth Enamel Is Composed of the Mineral Hydroxyapatite 10-59, but It Reacts with Weak Acids in the Mouth
Tooth enamel is composed of the mineral hydroxyapatite. It is essentially insoluble in water with Ksp = 2.3 10-59, but it reacts with weak acids in the mouth as described by one of the reaction equations below. You can determine the equilibrium constant, K, for this reaction from Ksp and Kw (the equilibrium constant for water autoionization) . How do you do this? 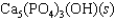
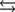
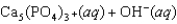
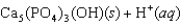
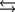
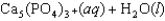
A) K = KwKsp
B) K = Kw /Ksp.
C) K = Ksp /Kw
D) K = Kw = Ksp
E) K = Kw = Ksp .
Correct Answer:
Verified
Q33: Which statement describing the mechanisms by which
Q34: Plants convert nitrogen into ammonia. This biological
Q35: Different concentrations of ions on either
Q36: Tooth enamel is composed of the calcium
Q37: Stomach acid is 0.16 M HCl. What
Q39: Enzymes in plants convert nitrate into ammonia.
Q40: Plants convert nitrogen into ammonia. This biological
Q41: Plants convert nitrogen into ammonia. This biological
Q42: Selenium can substitute for sulfur in the
Q43: Plants convert nitrogen into ammonia. This biological
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents