Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of the nitrite ion in the balanced reaction equation? 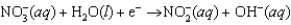
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:
Verified
Q47: Hydroxyapatite, which is a component of
Q48: Selenium is an ultratrace element that is
Q49: Chromium is an ultratrace metal in the
Q50: Adding fluoride ions to toothpaste and drinking
Q51: What is the dominant role played by
Q53: Plants react urea with water to produce
Q54: Chromium is an ultratrace metal in
Q55: Adding fluoride ions to toothpaste and drinking
Q56: Iodine's role is very specific. It is
Q57: Enzymes in plants convert nitrate into ammonia.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents